Clinical statistics show that 5-10% of fractures will lead to serious bone defects that cannot heal themselves [1]. The gold standard for repairing such defects is autologous bone grafts. However, this solution is not ideal. The bone taken from the body requires two or more operations. The amount of bone is limited, and there are risks of infection and pain in the donor area. The 3D printing scaffold made by additive manufacturing technology can allow blood flow and bone tissue growth through its porous structure design. The adjustment of its process parameters can replicate the anisotropy of bone to a certain extent. The precise 3D image of bone defect obtained by tomography or magnetic resonance imaging technology can also produce bone implants that fit the shape of the patient's bone defect. In the past few years, additive manufacturing technology has become an emerging technology in the biomedical research field, especially in the field of bone tissue regeneration. Before the advent of additive manufacturing technology, traditional technologies (such as sol gel method, gas foaming method or freeze drying method) could also realize the porous design of bone scaffold materials, but these technologies could not guarantee the repeatability of 3D scaffold structure. The stable and controllable porous structure with high connectivity is an important factor to realize cell migration, adhesion and proliferation. Through this technology, the procedure of comparing the shape of the patient's bone defect - adjusting the shape of the implant can be moved to the preoperative stage, thus greatly reducing the operation time for doctors to adjust the implant during the operation, thereby reducing the operation risk. Therefore, additive manufacturing technology is suitable for application in personalized medical treatment, especially in the case of bone defect with very complex shape caused by trauma or tumor resection.
Before designing personalized bone defect substitutes for patients through additive manufacturing technology, we should first select appropriate materials as the 'cornerstone'. Generally speaking, six important criteria need to be considered in the selection of materials in bone tissue engineering: 1) Bionics, that is, to simulate the characteristics of natural bone as much as possible, and to have good biocompatibility; 2) Mechanical properties, namely elastic modulus and compressive stiffness, should be sufficient to support bone growth, but not too high to cause stress shielding; 3) Long-term biodegradability. Under ideal conditions, the scaffold should be gradually replaced by new bone; 4) It is easy to image, which is generally achieved through the contrast between bone and scaffold material, which is conducive to tracking and positioning after surgery and observing the effect of bone growth; 5) If necessary, it should be easy to cut tissue sections; 6) It is sterile and the sterilization process does not affect the function of the scaffold material. The fifth standard is mainly used in preclinical studies. Through tissue section observation, the quality, maturity, type, rejection reaction, formation of new blood vessels and the interaction between surrounding tissues and scaffolds can be evaluated (that is, whether they contact with the surface of scaffolds, whether they penetrate into pores, etc.). Although the sixth standard is often ignored, the sterilization process is very important for porous design.
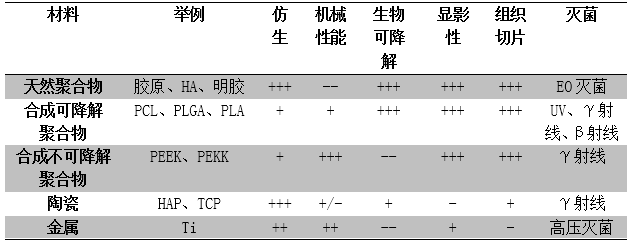
In terms of material types, there are currently four kinds of materials that can be used as bone defect substitutes: polymer, ceramic, polymer and ceramic composite, and metal. Table 1 shows the characteristics of the above four materials under the above six important standards.
Table 1: Analyze the properties of materials according to six standards: bionics, mechanical properties, biodegradability, development, tissue sectioning for preclinical research and applicable sterilization methods. From -- (very poor) to++(excellent performance).
1、 Polymer
(1) Natural polymer
Polymers can be divided into natural polymers and synthetic polymers. Natural polymers, such as chitosan, collagen, hyaluronic acid, gelatin or silk fibroin, have excellent biocompatibility and biodegradability. Their degradation cycle is usually within 2-24 weeks and will not produce any acidic by-products. However, due to their weak mechanical properties, they can not bear the force exerted on the bone, so these materials are mainly used as additives or part of composite materials to play their osteoinductive properties and enhance the ability of nuclear protein adhesion. This material is usually sterilized with relatively low temperature and mild ethylene oxide, and it is not recommended to choose electron beam irradiation or high-pressure sterilization. Electron beam irradiation will accelerate the degradation rate of polymer, increase the chemical crosslinking of materials, and high-pressure sterilization will reduce the viscosity and mechanical strength of materials.
(2) Synthetic degradable polymer
Synthetic degradable polymers usually include PCL, PLGA, PLA, PPF, etc. Their degradation rate can be adjusted, usually more than one year or two years, and they also have good biocompatibility and bone conductivity. Compared with the compressive strength (2-12MPa) and compressive modulus (52-318MPa) of natural bone, they have good mechanical properties. The compressive strength is about 2-39MPa (depending on porosity). At the same time, their radiopability, light weight and histocompatibility are conducive to preclinical research, but the material itself has no function of inducing bone formation. For this kind of material, high-pressure sterilization, ethylene oxide sterilization and plasma sterilization should be avoided. It is recommended to use ultraviolet radiation γ Ray or β Sterilization by radiation.
(3) Synthesis of non-degradable polymer
Non-degradable polymers, such as PEEK, PEKK, etc., have a compressive modulus range of about 0.14-8.21 GPa and a compressive strength of about 25-200 MPa according to different porosity. Like other polymers, they can also transmit radiation and have biocompatibility. However, such materials have poor interaction with cells or growth factors and low bone integration rate (that is, poor integration of scaffold and host bone). Therefore, titanium or hydroxyapatite coating is also sprayed on the surface to achieve surface modification and improve bone integration. In general, the main sterilization technologies that can be applied to non-degradable polymers are γ Radiation sterilization, because autoclaving may cause changes in physical and chemical properties of materials. It should be noted that this kind of material is non-degradable, and removal of material by surgery is an additional invasive risk for patients.
2、 Ceramics
Ceramic is a widely studied substitute material for bone defects, which is similar to the inorganic phase of natural bone, and has excellent bionic properties - both bone conductivity and bone induction. In bone tissue engineering, the most widely used ceramics are calcium phosphate, namely hydroxyapatite (HA) and tricalcium phosphate (TCP). They can be biodegradable, but the degradation rate is different. The compressive strength of ceramics ranges from 3-18 MPa, which is relatively weak and not suitable for bearing. Because the composition and density of ceramics are similar to that of primary bone, it is difficult to distinguish ceramics from primary bone on image, which also makes the histological analysis more complex. Generally speaking, ceramics can be used to repair various defects, and can also be used as scaffold filler or coating to enhance the biological activity of the scaffold. γ Radiation sterilization is generally the best sterilization process for ceramics.
3、 Polymer and ceramic composites
In order to optimize the mechanical and biological properties of bone defect substitutes, polymer and ceramic composite materials came into being, and the most widely used composite material is PCL/ β- Tricalcium phosphate( β- TCP)。 The degradation rate of PCL is very slow, so add β- TCP can achieve higher degradation rate, better compression performance and stronger bone induction.
4、 Metal
The most widely used metals in bone tissue engineering are titanium, titanium alloy and stainless steel, which are non-degradable metals. These materials have good biocompatibility, and their mechanical properties are close to or higher than those of natural bones (compression modulus is about 5-35GPa, compression strength is about 50-325MPa). It should be noted that in clinical use, high elastic modulus may produce stress shielding; Metal ions released during wear or corrosion may also lead to tissue absorption or necrosis; Its high hardness is also not conducive to pre-clinical tissue section research.
The treatment of bone defects, especially critical size bone defects, is still challenging. In order to meet clinical needs, bone replacement implants should have the following characteristics: 1) good biocompatibility, preferably biodegradable materials; 2) It has a porous structure that allows cells and body fluids to penetrate, but at the same time has enough mechanical strength to resist physiological load; 3) Match the shape of the bone defect; 4) Easy to process; 5) It is easy for doctors to operate. Additive manufacturing technology provides the possibility of providing customized bone defect replacement scaffold, which has a perfect external shape for a given defect and an internal structure conducive to bone repair. The combination of additive manufacturing technology and suitable materials can effectively control the mechanical and biological characteristics of bone defect substitutes.
reference:
[1] Garot C , Bettega G , Picart C . Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics[J]. Advanced Functional Materials, 2020.