Recently, the State Drug Administration issued Announcement No. 30 in 2022 to adjust part of the contents of the Classification Catalogue of Medical Devices, and the 'tissue inducible implant device' was officially listed as a newly added subdirectory (13-11-04). This is Academician Zhang Xingdong and his team who voted for the 'tissue inducible biomaterials' to be included in the 'definition of biomaterials in the 21st century' in 2018; In 2021, after passing the project approval application, forming the draft for approval and the national recommended standard of 'bone-induced calcium phosphate bioceramics' in the process of approval; International original achievements in the regulatory science field of 'tissue inducible biomaterial products' again!
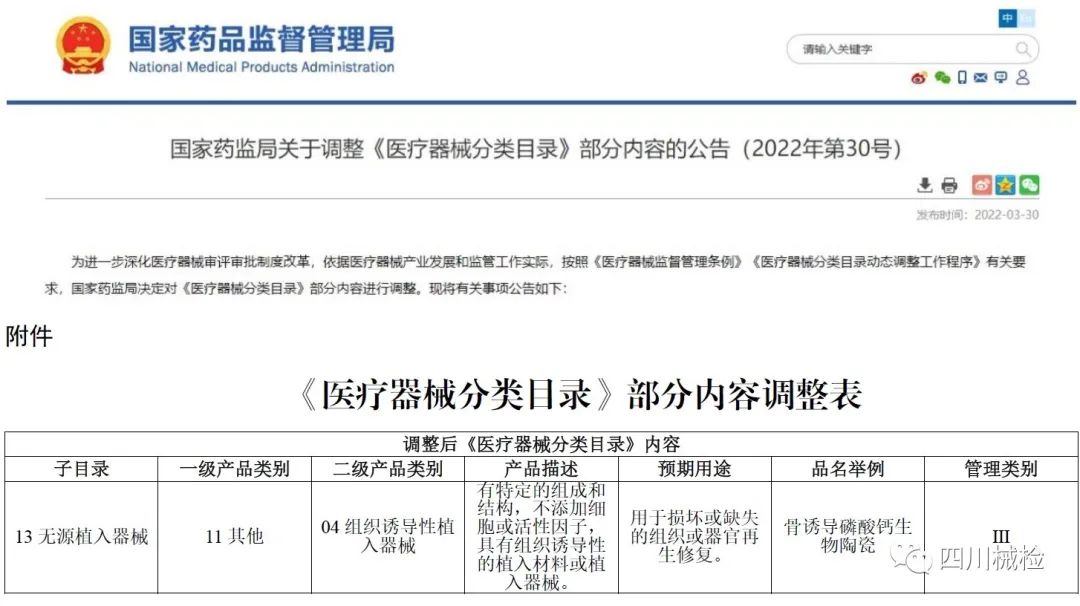
Image information source: https://www.nmpa.gov.cn/directory/web/nmpa/ylqx/ylqxggtg/20220330144627167.html
In the early 1990s, Academician Zhang Xingdong and others reported the ectopic osteogenesis of calcium phosphate ceramics without any factor or cell loading in dogs and other animals, indicating that biomaterials can also induce bone formation without adding any cell and growth factor, and thus proposed the bone induction mechanism. In 2003, the internationally pioneered osteoinductive artificial bone product developed by Academician Zhang Xingdong's team was successfully approved for the national Class III medical device product registration certificate and widely used in clinical practice, with good repair effect. As a major scientific achievement independently researched and developed by China, the research related to osteoinductive biomaterials won the second prize of the National Natural Science Award in 2007. In 2018, it was selected as the 'Great Change - Celebration of the 40th Anniversary of Reform and Opening up' large-scale exhibition, which was exhibited in the fourth unit of the 'Meteorology of Great Countries' in the fifth district, 'Scientific and technological innovation to support the dream of a powerful country'. The related research on the phenomenon and mechanism of bone induction also provides important guidance for the formation of other tissues induced by materials. The cartilage-induced collagen-based cartilage repair matrix product, which was initiated internationally by academician Zhang Xingdong's team, also successfully passed the special approval application of the National Drug Administration (NMPA) for innovative medical devices in 2021.
The regulatory science of medical products is the science of researching and developing new tools, new standards, new methods, new technologies and new approaches for evaluating the safety, effectiveness, quality and performance of regulated medical products in their whole life cycle. Our university is the first to establish a scientific research institute of medical device supervision in the country, and has become a leader in domestic research in this field. The Institute has made a lot of progress in organizing regulatory scientific research on inducible biomaterial products and the establishment of regulatory scientific system. For example, research projects related to tissue induced regeneration biomaterial products were listed in the first and second batch of China's drug regulatory scientific action plans issued by the State Drug Administration, and the 'Key Laboratory for Quality Research and Control of Tissue Induced Regeneration Biomaterials' led by the Institute was also approved by the State Drug Administration in 2021. At the same time, the research institute is also actively organizing the formulation of the guiding principles for the technical review of the group standards and product registration related to inducible biomaterials. With the strong promotion of the school, the science of medical product regulation in China will be further developed, and will promote the technical transformation and clinical application of a number of innovative biomaterial products, including tissue inducible biomaterials.